Cctv news: According to the official WeChat of the State Administration of Market Supervision, WeChat official account News, tea and related products, dairy products, beverages, alcohol, cakes, roasted seeds and nuts, and biscuits … … Are these foods that we often eat safe? Recently, the General Administration of Market Supervision organized food safety supervision and sampling inspection.
This sampling inspection includes processed grain products, edible agricultural products, tea and related products, dairy products, alcohol, cakes, potatoes and puffed foods, egg products, bean products, bee products, canned vegetables, fruit products, meat products, condiments, frozen drinks, infant formula foods, special dietary foods and edible oils, fats and their products.
Involving 663 batches of samples of 19 categories of food.:
Among them, 7 batches of samples of two categories of food, such as infant formula food and edible agricultural products, were found to be unqualified.
The main problems found are microbial pollution and excessive residues of agricultural and veterinary drugs.
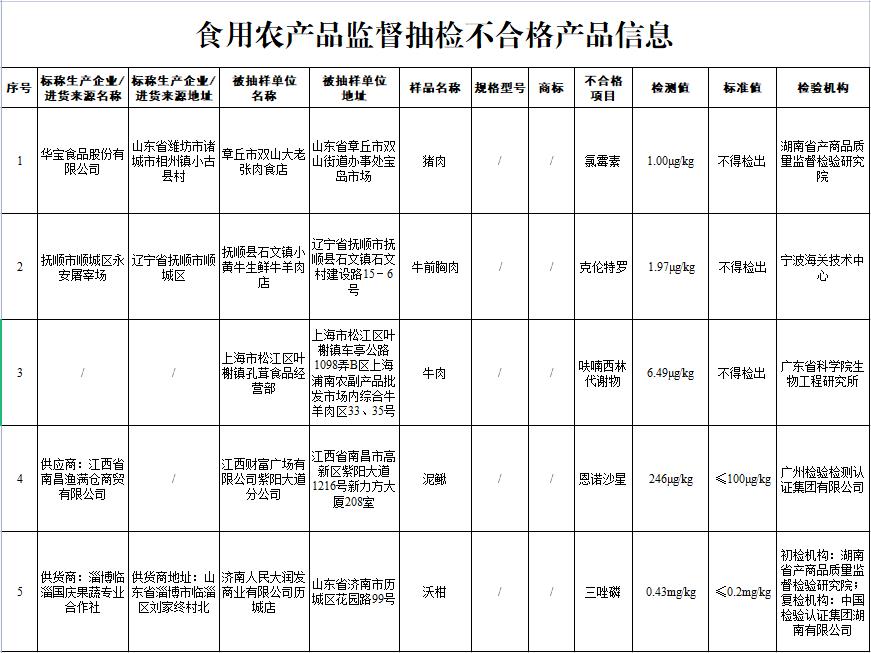
Unqualified food found in sampling inspection:
The General Administration of Market Supervision has instructed provincial market supervision departments such as Liaoning, Heilongjiang, Shanghai, Jiangxi and Shandong to immediately organize verification and disposal, find out the product flow, and urge enterprises to take measures such as recalling unqualified products from the shelves to control risks; Strictly deal with violations of laws and regulations according to law; Timely disclose the risk prevention and control measures and verification and disposal of enterprises to the public, and report to the General Administration.
Specific situation
I. Microbial pollution
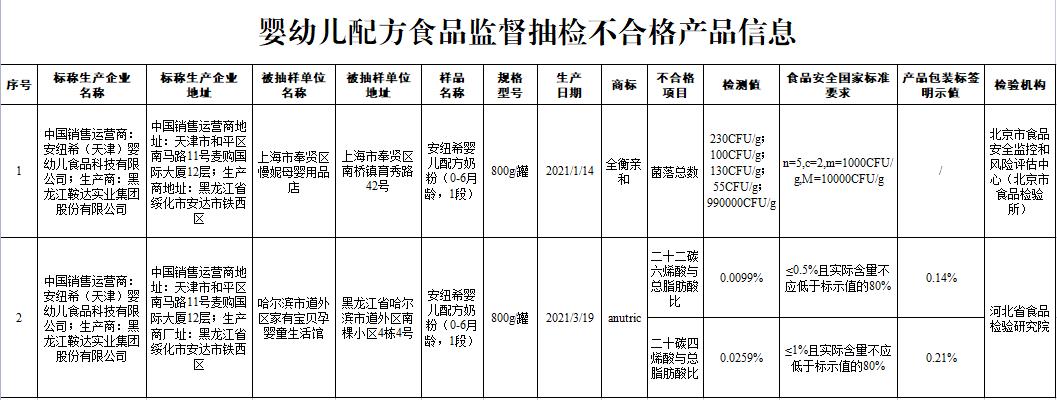
Anniuxi infant formula milk powder (0-6 months old, stage 1) manufactured by Heilongjiang Anda Industrial Group Co., Ltd. and sold and operated by Anniuxi (Tianjin) Infant Food Technology Co., Ltd. in China, the total number of detected colonies did not meet the national food safety standards, and the ratio of docosahexaenoic acid to total fatty acid and the ratio of eicosatetraenoic acid to total fatty acid did not meet the product labeling requirements. The inspection institutions are Beijing Food Safety Monitoring and Risk Assessment Center and Hebei Food Inspection and Research Institute. The Market Supervision Bureau of Suihua City, Heilongjiang Province urged enterprises to recall relevant products immediately, demanding that enterprises suspend business for rectification, confiscate illegal income of 18.4536 yuan and impose a fine of 872,000 yuan. Shanghai Municipal Market Supervision Bureau imposed an administrative penalty on the unit that sold unqualified products, Manni Maternal and Child Supplies Store in Fengxian District, Shanghai, confiscating illegal income of 1,736 yuan and fined 50,000 yuan.
Second, the problem of excessive residues of agricultural and veterinary drugs
(1) Pork from Shandong Huabao Food Co., Ltd. sold by Shuangshanda Laozhang Meat Store in Zhangqiu City, Shandong Province, in which chloramphenicol residue does not meet the national food safety standards. The inspection institution is Hunan Institute of Commodity Quality Supervision and Inspection.
(2) Cattle breast meat from Yong ‘an Slaughterhouse, Shuncheng District, Fushun City, Liaoning Province, which was sold by Xiaowen Town, Fushun County, Fushun City, Liaoning Province, and the clenbuterol residue did not meet the national food safety standards. The inspection agency is Ningbo Customs Technology Center.
(3) The residues of nitrofurazone metabolites in beef sold by the business department of Kongrong Food in Yexie Town, Songjiang District, Shanghai do not meet the national food safety standards. The inspection institution is the Institute of Bioengineering, Guangdong Academy of Sciences.
(4) Misgurnus anguillicaudatus sold by Ziyang Avenue Branch of Jiangxi Fortune Plaza Co., Ltd. and from Man Cang Trading Co., Ltd. in Nanchang, Jiangxi Province, in which the residue of enrofloxacin does not meet the national food safety standards. The inspection organization is Guangzhou Inspection and Certification Group Co., Ltd..
(5) Wogan sold by Licheng Store of Jinan People’s RT Mart Commercial Co., Ltd. in Shandong Province and from Linzi Guoqing Fruit and Vegetable Professional Cooperative in Zibo, Shandong Province, was found by the Hunan Provincial Institute of Commodity Quality Supervision and Inspection that the triazophos residue did not meet the national food safety standards. Licheng Store of Jinan People’s RT Mart Commercial Co., Ltd. raised objections to the inspection results and applied for re-inspection; After re-inspection by China Inspection and Certification Group Hunan Co., Ltd., the preliminary inspection conclusion was maintained.
Annex 1
Small knowledge of some unqualified inspection items
First, the total number of colonies
The total number of colonies is an indicative microbial index, not a pathogen index, which reflects the hygienic status of food in the production process. Infants and young children have low immunity, belonging to susceptible population, and eating food contaminated by microorganisms with poor sanitary quality can easily lead to vomiting and diarrhea. National Standard for Food Safety Infant Formula (GB 10765— In 2010), it is stipulated that the test results of the total number of colonies of five samples of the same batch of infant formula food should not exceed 10000CFU/g, and the test results of two samples are allowed to exceed 1000CFU/g at most. The reason why the total number of colonies in infant formula food exceeds the standard may be that the enterprise does not strictly control the sanitary conditions in the production and processing process as required, or it may be related to the lax packaging and sealing of the products or improper storage and transportation conditions.
Second, chloramphenicol
Chloramphenicol is an amide alcohol antibiotic, which has a good inhibitory effect on both gram-positive and gram-negative bacteria. Chloramphenicol residues generally do not cause acute poisoning; Long-term intake of foods with excessive chloramphenicol residues may accumulate in the human body, resulting in drug resistance and cross-resistance to similar drugs, causing gastrointestinal symptoms, abnormal liver function and abnormal blood system. The List of Drugs and Other Compounds Prohibited in Food Animals (Announcement No.250 of the Ministry of Agriculture and Rural Affairs) stipulates that chloramphenicol is a drug prohibited in food animals (it cannot be detected in animal foods). The reason for the detection of chloramphenicol in pork may be the illegal use in the breeding process.
Third, Clenbuterol
Clenbuterol belongs to β -Stimulants can promote protein deposition in animals, promote fat decomposition and inhibit fat deposition, improve lean meat rate and gain weight, so they are called "lean meat". Long-term consumption of animal food with clenbuterol may cause skeletal muscle tremor, palpitation, tachycardia, fatigue, headache, nausea and dyspnea in limbs, face and neck. It is stipulated in the List of Non-edible Substances and Food Additives Easily Abused in Food (Fourth Batch) (Rectification Office Letter [2010] No.50), β -Stimulant drugs (clenbuterol hydrochloride, ractopamine, etc.) are non-edible substances that may be illegally added in food (not detectable in animal food). The reason why clenbuterol was detected in beef may be that it was used illegally in order to improve lean meat rate, weight gain and feed conversion rate in the breeding process.
IV. Metabolites of Furacillin
Furacillin is a nitrofuran antibacterial agent, which has the characteristics of broad antibacterial spectrum and has been widely used in livestock and poultry and aquaculture. Nitrofurans are rapidly metabolized in organisms, and their metabolites are stable after binding with protein. Therefore, it is necessary to detect their metabolites to reflect the residual status of nitrofurans. Long-term consumption of a large number of foods with nitrofurazone metabolites may accumulate in the human body, causing allergic reactions, gastrointestinal reactions, multiple peripheral neuritis and so on. The List of Drugs and Other Compounds Prohibited in Food Animals (Announcement No.250 of the Ministry of Agriculture and Rural Affairs) stipulates that furacilin is a drug prohibited in food animals (it cannot be detected in animal foods). The reason for the detection of nitrofurazone metabolites in beef may be the illegal use in the process of breeding.
V enrofloxacin
Enrofloxacin belongs to the third generation of quinolones, which is a kind of synthetic broad-spectrum antibacterial drugs. It is used to treat skin infections and respiratory infections in animals, and it is an exclusive drug for animals. Long-term consumption of food with excessive residues of enrofloxacin may accumulate in the human body, which may harm human function and may also cause drug-resistant strains in the human body. Maximum Residue Limits of Veterinary Drugs in Food (GB 31650— 2019) stipulates that the maximum residue limit of enrofloxacin in fish skin and meat is 100μ g/kg。 The reason why enrofloxacin residues in Misgurnus anguillicaudatus exceed the standard may be that the drug residues in the products on the market exceed the standard because of increasing the dosage illegally or not observing the drug withdrawal period in order to control the epidemic disease quickly in the breeding process.
Six, triazophos
Triazophos is an organophosphorus pesticide, which has contact toxicity and stomach toxicity, and has good control effect on citrus red spider. A small amount of residue will not cause acute poisoning, but long-term consumption of food with excessive triazophos may have a certain impact on human health. Maximum Residue Limits of Pesticides in Food (GB 2763— 2019) stipulates that the maximum residue limit of triazophos in citrus is 0.2mg/kg. The reason why triazophos residues in citrus exceed the standard may be that in order to control pests quickly, the dosage of triazophos is increased or the picking interval is not observed, which leads to the excessive residues in products on the market.
7. ratio of docosahexaenoic acid to total fatty acids
Docosahexaenoic acid (DHA) is an essential polyunsaturated fatty acid, which participates in many physiological functions of the body and contributes to the development of intelligence and vision of infants. Lack or excess of docosahexaenoic acid may have a certain impact on the growth and development of infants. National Standard for Food Safety Infant Formula (GB 10765— In 2010), the ratio of docosahexaenoic acid to total fatty acids should not exceed 0.5%, and the national food safety standard pre-packaged foods for special dietary uses label (GB 13432— 2013) stipulates that the actual content of energy and nutrients should not be less than 80% of the marked value during the shelf life of the product. The ratio of docosahexaenoic acid to total fatty acids in this unqualified sample conforms to the National Standard for Food Safety Infant Formula (GB 10765— 2010) but does not meet the product labeling requirements, and the actual detection value is lower than 80% of the labeling value. The reason why the ratio of docosahexaenoic acid to total fatty acids in infant formula food is not up to standard may be that the actual addition amount of nutrients in the production process of enterprises is lower than that in the formula design; It may also be lost during processing or storage; It may also be related to the uneven distribution of related nutrients in the product due to the inadequate mixing process of the production process.
Eight, eicosatetraenoic acid and total fatty acid ratio
Eicosatetraenoic acid, AA or ARA, is a kind of polyunsaturated fatty acid, which is of great significance to the growth and development of infants and the improvement of brain and retina functions. The lack or excess of eicosatetraenoic acid may have a certain impact on the growth and development of infants. National Standard for Food Safety Infant Formula (GB 10765— In 2010), the ratio of eicosatetraenoic acid to total fatty acid should not exceed 1%, and the national food safety standard pre-packaged foods for special dietary uses label (GB 13432— 2013) stipulates that the actual content of energy and nutrients should not be less than 80% of the marked value during the shelf life of the product. The ratio of eicosatetraenoic acid to total fatty acids in this unqualified sample conforms to the National Standard for Food Safety Infant Formula (GB 10765— 2010) but does not meet the product labeling requirements, and the actual detection value is lower than 80% of the labeling value. The reason why the ratio of eicosatetraenoic acid to total fatty acid in infant formula food is not up to standard may be that the actual addition amount of nutrients in the production process of enterprises is lower than that in the formula design; It may also be lost during processing or storage; It may also be related to the uneven distribution of related nutrients in the product due to the inadequate mixing process of the production process.
Annex 2
Annex 3